|
|
Original article
THERAPY OF FEBRILE NEUTROPENIA IN PATIENTS WITH MALIGANCY
-single center expirience and recommendationes-
Goran Marjanović1, Vesna Marjanović2, Mladen Milenović1, Lana Mačukanović-Golubović1, Tomislav Vukićević1
1 Clinic of Hematology and Clinical Immunology ,Clinical Centre Nis
2 Clinic of Child Surgery and Orthophedia, Clinical Centre Nis
Introduction and Definitions
Myelosuppression and resulting neutropenia are very common in patients with malignancies undergoing hemotherapy. When the neutrophil count decreases to <1000 cells/mm3, the increased susceptibility to infection can be expected.
Fever is defined as a single oral temperature of >38.3°C or a temperature of >38.0°C for >1 h.
Neutropenia is defined as a neutrophil count of <500 cells/mm3, or a count of <1000 cells/mm3 with a predicted decrease to <500 cells/mm3. Increased susceptibility to infection is inversely proportional to neutrophil count. At least one-half of neutropenic patients who become febrile have an established or occult infection, and at least one-fifth of patients with neutrophil counts of <100 cells/mm3 have bacteremia.. A low nadir in the neutrophil count and protracted neutropenia are major risk factors for impending infection In addition to quantitative changes in neutrophil counts, abnormalities of phagocytic function or other deficits in the immune response may further increase the risk for infection in a neutropenic host.
Most Common Causes of Febrile Episodes in Neutropenic Patients
Gram-positive bacteria now account for 60%70% of microbiologically documented infections, although the rate of gram-negative infections is increasing in some centers. Some of the gram-positive organisms may be methicillin resistant and, therefore, are susceptible only to vancomycin, teicoplanin and one of the latest-linezolid. These are often more indolent infections (e.g., infections due to coagulase-negative staphylococci, vancomycin-resistant enterococci, or Corynebacterium jeikeium), and a few days' delay in administration of specific therapy may not be detrimental to the patient's outcome, although it may prolong the duration of hospitalization. Other gram-positive bacteria (S. aureus, viridans streptococci, and pneumococci) may cause fulminant infections resulting in serious complications or death, if not treated promptly (12). Gram-negative bacilli, especially P. aeruginosa, Escherichia coli, and Klebsiella species, remain prominent causes of infection and must be treated with selected antibiotics (14). Although fungal infections are usually superinfections, in some cases, Candida species or other fungi can cause primary infections. Viral infections are not very often and are more common in heavily immunosupressed patients.
Recomendations for Initial Patient Evaluation
Initial evaluation should consist of a thorough physical examination; a complete blood cell count; measurement of serum levels of creatinine, urea nitrogen, and transaminases; and culture of blood samples for bacteria and fungi. A chest radiograph is indicated for patients with respiratory signs or symptoms or if outpatient management is planned. Some experts recommend "baseline" chest radiograph in patients with neutropenia with high risk for respiratory infections development.
The primary anatomic sites of infection often include the alimentary tract, where cancer chemotherapyinduced mucosal damage allows invasion of opportunistic organisms. Similarly, damage to the integument by invasive procedures, such as placement of vascular access devices, often provides portals of entry for infectious organisms Symptoms and signs of inflammation may be minimal or absent in the severely neutropenic patient, especially if accompanied by anemia. Diminished or absent induration, erythema, and pustulation in response to bacterial infection leave the patient with a cutaneous infection without typical cellulitis, a pulmonary infection without discernible infiltrate on a radiograph, meningitis without pleocytosis in the CSF, and a urinary tract infection without pyuria. Nevertheless, a search should be undertaken for subtle symptoms and signs, including pain at the sites most commonly infected. These sites are the periodontium; the pharynx; the lower esophagus; the lung; the perineum, including the anus and the skin around vascular catheter access sites including bone marrow aspiration sites.
Specimens should be obtained immediately for culture for bacteria and fungi. If a central venous access device is in place it is recomended for blood samples to be obtained for culture from the device lumen(s) as well as from a peripheral vein. If a catheter entry site is inflammed or draining, the fluid exuded should be examined by Gram staining.
Little clinically useful information is gained from performing routine cultures of samples from the anterior nares, oropharynx, urine, and rectum, when lesions or disease processes are absent. In case with previosly isolated: meticilin-resistant Staphylococcus aureus, penicilin-resistant pneumococci, or Aspergillus species, as well as multiresistant Pseudomonas aeruginosa, from rectal mucosa and vancomicin-resistant enterococci a frequent sample collection is necessery for infection-control purposes.
Initial evaluation answer following questions: (1) Does the specific patient belog to low risk group; (2) Is vancomycin therapy necessary?
Determination of Risk Groups in Neutropenic Patients
An assumption that hospitalization of a patient is the safest course to take is not necessarily correct. Outpatient treatment of low-risk episodes of fever and neutropenia is substantially less costly than inpatient care and is preferred by most patients and families.(4)
Factors determining risk levels for infective complications development are illustrated in a recent international collaborative study of 1139 febrile and neutropenic patients with malignancy. In this study a scoring system was established and validated in order to identify, at the time of presentation with fever, those patients with low risk for complications, including mortality. Factors associated with lower risk for complications and a higher rate of favorable outcome were as follows: age <60 years (children not included), cancer in partial or complete remission, no symptoms or only mild to moderate symptoms of illness, outpatient status at the time of onset of fever, temperature <39.0°C, normal findings on chest radiographs, absence of hypotension, respiratory rate of <24 breaths/min, absence of chronic pulmonary diseases, absence of diabetes mellitus, absence of confusion or other signs of mental status alteration, absence of blood loss, absence of dehydration, no history of fungal infection, and no receipt of antifungal therapy during the 6 months before presentation with fever. (10) Low risk-index score was developed based on 7 characteristics (table 1). A risk-index score of >21 identified low-risk patients with a positive predictive value of 91%, specificity of 68%, and sensitivity of 71%.
Table 1 Scoring index for identification of low-risk febrile neutropenic patients at time of presentation with fever.
|
Characteristic |
Score |
|
Extent of illness (choose only one item bellow) |
5 |
|
No symptoms |
5 |
|
Mild symptoms |
3 |
|
Moderate symptoms |
5 |
|
No hypotension |
4 |
|
No chronic obstructive pulmonary disease |
4 |
|
Solid tumor or no fungal infection |
3 |
|
No dehydration |
3 |
|
Outpatient at onset of fever Age <60 years |
2 2 |
Initial Antibiotic Therapy
Oral antibiotic treatment
This approach is appropriate for low-risk adults only; use ciprofloxacin plus amoxicillin-clavulanate.
Intravenous antibiotic treatment
There are three general schemes of intravenous antibiotic therapies with similar efficacy, with the caveat that one may be more appropriate for certain patients and in certain institutions than others. The schemes are as follows: (1) single-drug therapy (monotherapy),(2) 2-drug therapy without a glycopeptide (vancomycin), and (3) therapy with glycopeptide (vancomycin) plus 1 or 2 drugs. (Figure 1)
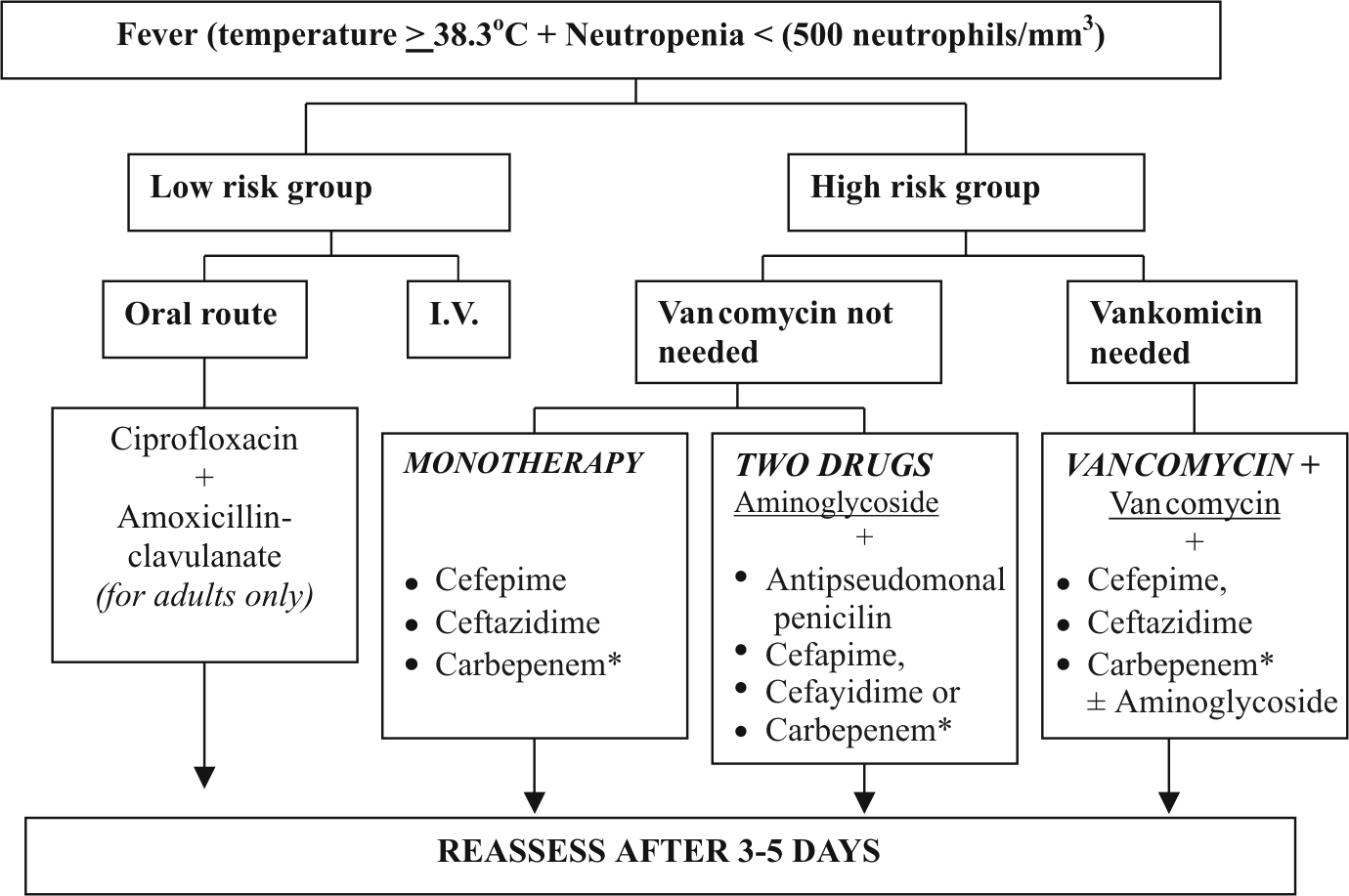
Figure 1 Algorithm for initial management of febrile neutropenic patients
* Carbapenem-Imipenem or Meropenem (8)
Monoherapy.
Recommendation- Choose therapy with 1 of the following agents: cefepime or ceftazidime, or imipenem or meropenem. Several studies have shown no striking differences between monotherapy and multidrug combinations for empirical treatment of uncomplicated episodes of fever in neutropenic patients. (1, 7, 15) A third- or fourth-generation cephalosporin (ceftazidime or cefepime) or a carbapenem (imipenem-cilastatin or meropenem) may be successfully used as monotherapy. (7.13) Physicians should be aware that extended-spectrum b-lactamases and type 1 b-lactamases have reduced the utility of ceftazidime for monotherapy. Cefepime, imipenem-cilastatin, and meropenem, unlike ceftazidime, have excellent activity against viridans streptococci and pneumococci. Vancomycin was shown to be less frequently required with cefepime than with ceftazidime monotherapy.
Two-drug therapy without a glycopeptide antibiotic (vancomycin).
Recommendation-Choose an aminoglycoside plus antipseudomonal penicillin, cephalosporin (cefepime or ceftazidime), or carbapenem. The most commonly used 2-drug therapy, excluding regimens with vancomycin, includes an aminoglycoside (gentamicin, tobramycin, or amikacin) with an antipseudomonal carboxypenicillin or ureidopenicillin (ticarcillin-clavulanic acid or piperacillin-tazobactam); an aminoglycoside with an antipseudomonal cephalosporin, such as cefepime or ceftazidime; and an aminoglycoside plus a carbapenem (imipenem-cilastatin or meropenem). It is important to have in mind similar efficacy of those combinations but also that the susceptibility of isolates to antibiotics used at the time of the study may be different from susceptibilities of some bacterial isolates today.
The advantages of combination therapy are potential synergistic effects against some gram-negative bacilli and minimal emergence of drug-resistant strains during treatment. (2) The major disadvantages are the lack of activity of these combinations, such as ceftazidime plus an aminoglycoside, against some gram-positive bacteria, and the nephrotoxicity, ototoxicity, and hypokalemia associated with aminoglycoside compounds and carboxypenicillins. Limited studies show that a single daily dose of an aminoglycoside with ceftriaxone is as effective as multiple daily doses of these drugs and as effective as monotherapy with ceftazidime. (9)
Vancomycin plus 1 or 2 antibiotics, if criteria for use of vancomycin are met.
Recommendation-Choose cefepime or ceftazidime plus vancomycin, with or without an aminoglycoside; carbapenem plus vancomycin, with or without an aminoglycoside; or antipseudomonal penicillin plus an aminoglycoside and vancomycin.
Inclusion of vancomycin in initial empirical therapy may be prudent for selected patients with the following clinical findings: (1) clinically suspected serious catheter-related infections (e.g., bacteremia, cellulitis), (2) known colonization with penicillin- and cephalosporin-resistant pneumococci or methicillin-resistant S. aureus, (3) positive results of blood culture for gram-positive bacteria before final identification and susceptibility testing, or (4) hypotension or other evidence of cardiovascular impairment. (3)
For some physicians in some medical centers, intensive chemotherapy that produces substantial mucosal damage (e.g., high-dose cytarabine) or increases the risk for penicillin-resistant streptococcal infections (e.g., infection with viridans streptococci), as well as prophylaxis with quinolones for afebrile neutropenic patients before the onset of fever, are also considered indications for vancomycin to be included in the initial regimen. Sudden increase of temperature to >40°C has, to some extent, been predictive of sepsis with viridans streptococci. (5)
Antibiotic Regimen Menagment During the First Week of Treatment
Estimation of antibiotic efectivity should be done after 3-5 days of initial regimen. From this point, decisions regarding further treatment are made on the basis of whether the patient had bacteremia or pneumonia, whether the fever has resolved, and whether the patient's condition has deteriorated. Some patients' conditions may deteriorate rapidly in <3 days, necessitating reassessment of the empirical regiment while.others that are stable could be reassessed beyond day 5. (9)
Patient is afebrile within 35 days of treatment. If a causative microbe is identified, the antibiotic regimen may be changed, if necessary, to provide optimal treatment with minimal adverse effects and lowest cost, but broad-spectrum coverage should be maintained to prevent breakthrough bacteremia. In the absence of discernible infectious disease (e.g., pneumonitis, enterocolitis, cecitis, endocarditis, catheter-associated infection, or severe cellulitis) and of positive culture results, treatment for compliant adults may be changed after >2 days of intravenous therapy to an oral antibiotic combination of ciprofloxacin and amoxicillin-clavulanic acid. Children who lack signs of sepsis (chills, hypotension, and requirement for fluid resuscitation) and severe mucositis at the time of admission and throughout their courses and who are at low risk for complications may have intravenous antibiotic treatment stopped and therapy continued with oral cefixime. Afebrile high-risk patients should continue parenteral therapy.
Persistent fever during first 3-5 days. Reassess therapy on the third day or no later than the fifth day. If there is no clinical worsening, continue use of the same antibiotics; stop vancomycin use if cultures do not yield organisms. If there is progressive disease, change antibiotics. If the patient is febrile after 5 days, consider adding an antifungal drug, with or without a change in antibiotic regimen. (Figure 2)
Duration of Antibiotic therapy
Patient is afebrile by day 3. If the patient's neutrophil count is >500 cells/mm3 for 2 consecutive days, if there is no definite site of infection, and if cultures do not yield positive results, stop antibiotic therapy when the patient is afebrile for >48 h. If the patient's neutrophil count is <500 cells/mm3 up to the seventh day, if the patient was initially at low risk, and if there are no subsequent complications, stop therapy when the patient is afebrile for 57 days. If the patient was initially at high risk and there are no subsequent complications, continue antibiotic therapy.
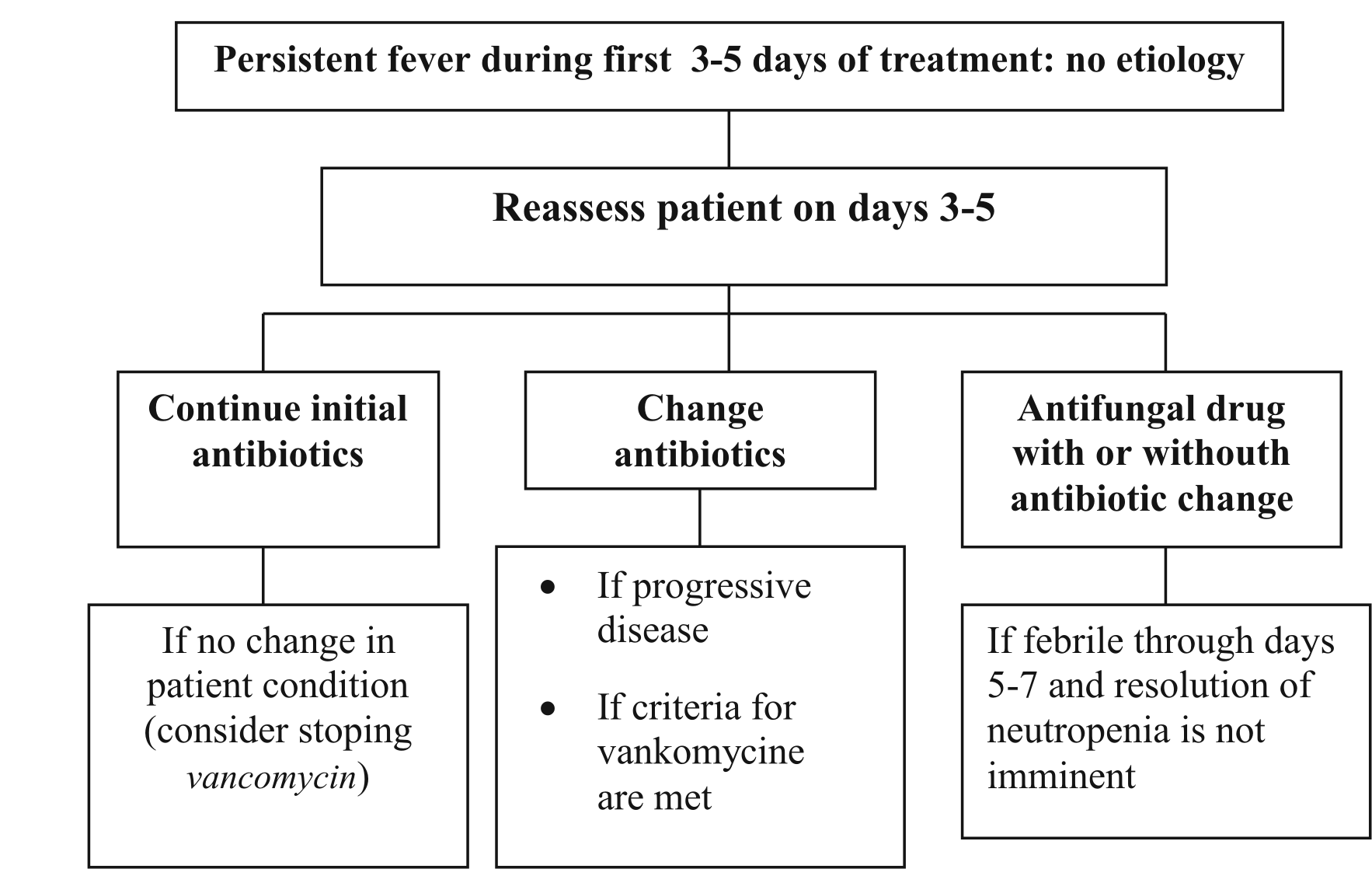
Figure 2 Guide to treatment of patients who have persistent fever after 35 days of treatment and for whom the cause of the fever is not found (8)
Persistent fever on day 3. If the patient's neutrophil count is >500 cells/mm3, stop antibiotic therapy 45 days after the neutrophil count is >500 cells/mm3. If the patient's neutrophil count is <500 cells/mm3, reassess and continue antibiotic therapy for 2 more weeks; reassess and consider terminating therapy if no disease site is found.
Use of Antiviral Drugs
Antiviral drugs are not recommended for routine use unless clinical or laboratory evidence of viral infection is evident..
Granulocyte Transfusions
Granulocyte transfusions are not recommended for routine use.
Use of Colony-Stimulating Factors
The use of colony-stimulating factors is not routine but should be considered in certain cases with predicted worsening of course. Guidlines of American Society of Clinical Oncology could also be recomended for making decidions. (11)
Antibiotic Prophylaxis for Afebrile Neutropenic Patients
Use of antibiotic prophylaxis is not routine because of emerging antibiotic resistance, except for the use of trimethoprim-sulfamethoxazole to prevent Pneumocystis carinii pneumonitis. Antifungal prophylaxis with fluconazole and antiviral prophylaxis with acyclovir or ganciclovir are warranted for patients undergoing allogenic hematopoietic stem cell transplantation..
The Aim of the Study
The aim of this study is to show our own experience in the treatment of febrile neutropenic patients who have severe immunosuppression due to chemotherapy and underlying myelosuppressive diseases. The guidelines for treatment options issued by Infectious Disease Society of America were given as well. (8)
Material and Methods
In this study 109 patients diagnosed and treated during the period from January 1st 2001 - 31st December 2002 at the Clinic of Hematology and clinical immunology Clinical centre Nis were analyzed. At the time of apearence of neutropenia after chemotherapy all patients were reassessed by thorough physical examination; a complete blood cell count; measurement of serum levels of creatinine, urea nitrogen, and transaminases; and culture of blood samples for bacteria and fungi (in some cases cultures were taken from empyema and piogenic wounds) as well as urine cultures in cases of clinical suspition for urinary infection. Chest radhiographs were routinely performed in all patients before initiation of chemotherapy with reassessment in clinical conditiones suggesting respiratory infections. Abdomen CT scans were done in suspitious casses for viscous and retroperitoneal infections.
Initial empirical antibiotic therapy at Clinic of Hematology Nis consists of trimethoprim-sulfamethoxazole, aminoglycoside and usually cefalosporine of the second generation. Second line therapy is consisted of ciprofloksacine, ceftazidime and vankomycine and with culture results addition of the most appropriate antibiotic. According to ASCO guidlines colony-stimulating factors are given to patients where profound and prolonged neutropenia is expected. (11) Local therapy of oral cavity for treatemnt of lesions of mucosae as well as uroprtection in case if osfomide administration is performed.
Results
The largest number of patients-38 were treated of acute leukemia, while 29 suffered of Non Hodgkin's lymphoma (NHL), 18 had Hodgkin's disease(HD) and 24 had Multiple Myeloma (MM). Febrile neutropenia was recorded in 100% leukemia patients who recived chemotherapy, while in NHL group there were only 2 (6,8%). In the Myeloma group only 2 (8,44%) out of 24 patients and 2 out of 20 (10%) with HD had febrile neutropenia. (Figure 3). Cultures were positive in only 11 patients (table 2).
Table 2 Microbiological characteristics of febrile neutropenic hematological patients
|
Cause |
Isolated as sole (No. of casess) |
Mixed (No. of casess) |
Cause of death (No. of casess) |
Drug sensitivity |
|
|
Staphilococcus epidermidis |
6 |
2 |
2 * |
Vancomycin, Fucidinc acid Rifampicine
|
|
|
Staphilococcuus aureus |
|
2 |
1 |
Vancomycin |
|
|
Enterococcus faecalis |
|
2 |
|
Imipenem |
|
|
Enterobacter spp |
|
1 |
|
Amikacin Ciprofloxacin
|
|
|
E colli |
|
1 |
1 |
Ciprofloxacin |
|
|
Acinetobacter |
|
1 |
|
Ciprofloxacin |
|
|
Serratia spp |
|
1 |
1 |
Ciprofloxacin |
|
|
Pseudomonas aeroginosa |
|
1 |
1 |
Imipenem |
|
|
Candida albicans |
1 |
|
|
Flucconasol |
|
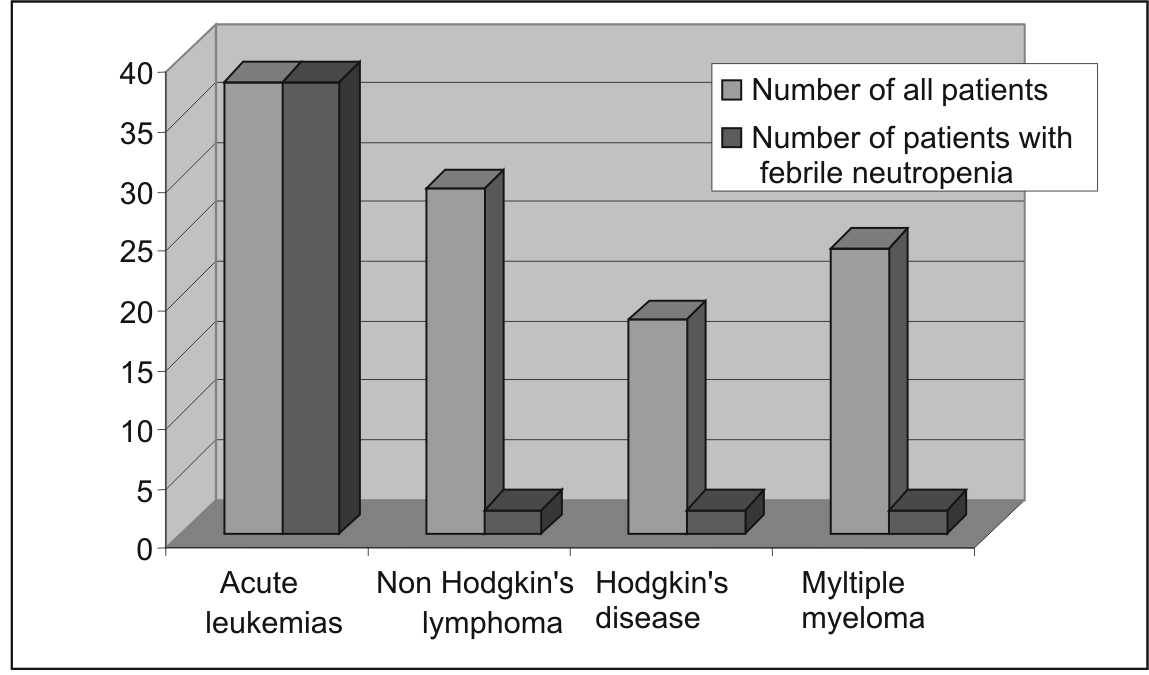
Figure 3 Incidence of febrile neutropenia according to hematological malignacy (see text)
Among patients with positive cultures 5 had acute leukemia, 2 sufered of NHL (T-immunophenotipes both), 2 and another 2 Myeloma and Hodgkin's disease, respectively. After sucssesfyl treatment of Staphilococcus epidermidis infection one patient with HD developed superinfection with Candida albicans.
Staphilococcus epidermidis was present in 72,7% cases, being only casuative agent isolated in > 50% (6 od 11) of patients. (table 2). Susceptibility analyzis of isolated Staphilococci species showed multidrugresistance to most of cephalosporines used at our clinic including ceftriaksone long with resistance to macrolides, penicilins, linkomycin. All gram-positive bacteria were sensitive to vankomycin. (table 2).
Gram-negative bacteria isolated from our patients were present only in mixed infections accompanied by gram-positive bacteria in several immunocomprimised cases. It is important to point out sensitivity of gram-negative bacteria to ciprofloxacine (table 2).
Death resulted from sepsis occured in 3 out of 11 patients described in this study. Staphilococcus epidermidis was only isolated microorganisam in two patient died of septicaemia while Pseudomonas Aeroginosa accompanied by other gram-negative bacteria and resistant Staphilococcus aureuswas was a cause of death in one severly immunocopromised case.(table 2). Later complications developed after sepsis withdrawal were empyema caused by Staphilococcus epidermidis and liver microapsceses registered in two patients, respectively. (Figure 4).
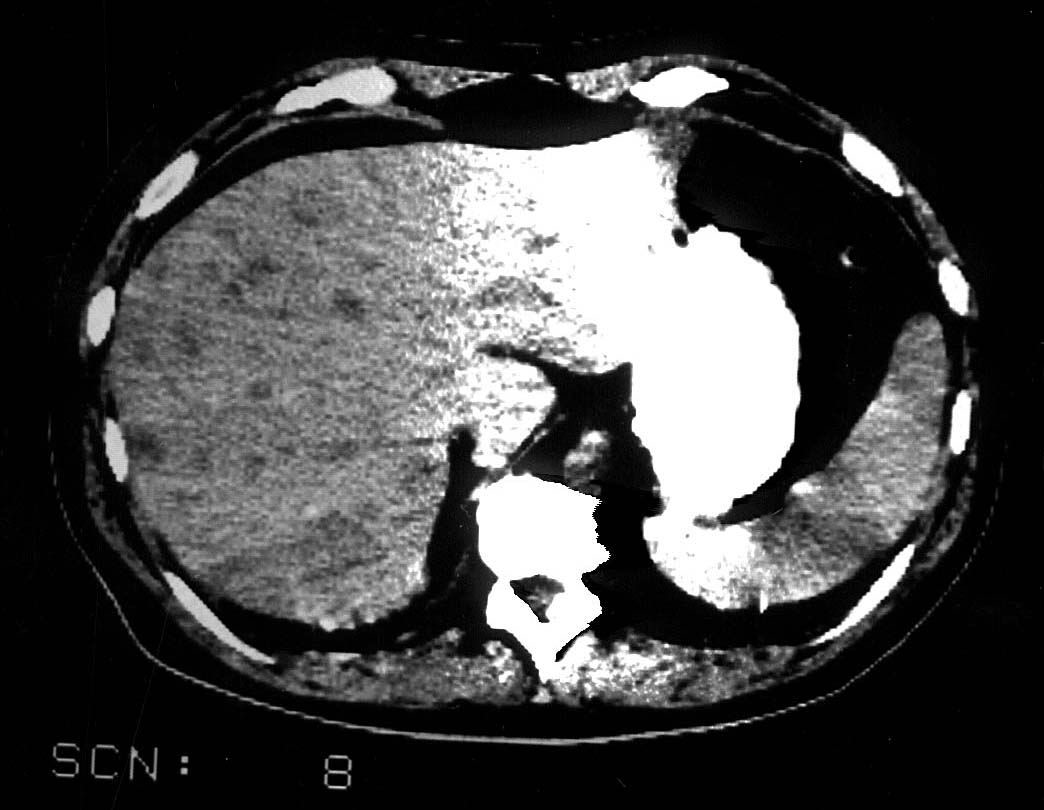
Figure 4. Intrahepatic apscesses in patient with febrile neutropenia
Discussion
Myelosuppression and resulting neutropenia are very common in patients suffering of hematological malignancies and especially of acute leukemia where trensitory bone marrow aplasia is one of the goals of chemotherapy. Mucous mebrane deterioration are also very common in patients with accute leukemias and relapsed or chemoresistant lymphomas, receiving high dose cytosin-arabinoside. All those factors lead to incresed sucsseptibility of patients towards infection putting them in group with high risk ones. Therfore, patients with leukemia should be initially treated with empirical parenteral therapy. This is the case with patients in our institution that receive combination of two antibiotics but withouth initial vankomycin. This strategy is based on previous reports from European Organization for Research and Treatment of Cancer stating that vankomycin is not necessery for initial empirical antibiotic therapy if it is readily available. (6) Excessive use of vankomycin could also result in emergence of resistant enterococci.
Our study clearly denies usefulness of those arguments to our patients. Incidence of gram-positive bacteria with 72,7% is comparable with a fact that gram-positive bacteria accounts for 60%70% of microbiologically documented infections. (8) Gram-positive organisms isolated at our center were susceptible only to vancomycin. At institutions like ours where these gram-positive bacteria are common causes of serious infections, vancomycin may be incorporated into initial therapeutic regimens of some high-risk patients but discontinued 24-48 hours later if no such infection is identified in cultures.
Almost all isolated gram-negative organisms at our instituion were supsetible to quinolones due to their uncommon use in antibiotic prophilaxis of fever in neutropenic patients. Another reason lies in the fact that quinolones are given in combinations rather than as a monotherapy in order to prevent drug resistance, as well as due to their limited spectrum of efectivnes.
Economic issues and the fact that the vast majority of standard therapeutic approaches for hematological neoplasms, besides accute leukemias, are rarely accompanied with febrile neutropenia, favour outpatient treatment aproach, oral antibiotic therapy and antibiotic prophilaxis.
Conclusion
In the selection of the initial antibiotic regimen, one should consider the type, frequency of occurrence, and antibiotic susceptibility of bacterial isolates recovered from other patients at the same hospital.
Outpatient approach with oral antibiotics for low risk group of patients should be apllied whenever possible. Hospital tretment is mandatory for all accute leukemia patients with initial empirical antibiotic therapy aproach consisted of 2 parenteral drugs with the addition of vankomycine.
We emphasize that no specific scheme, no specific drug or combination of drugs, and no specific period of treatment can be unequivocally applied to all febrile neutropenic patients. It is important to note that the guidelines are general and must be applied wisely with respect to variations in individual patients and types of infections, settings in which patients are being treated, antimicrobial susceptibility patterns, underlying causes of neutropenia, and expected time of recovery.
Literature:
Behre G, Link H, Maschmeyer G, et al. Meropenem monotherapy versus combination therapy with ceftazidime and amikacin for empirical treatment of febrile neutropenic patients. Ann Hematol 1998; 76:7380
Brown AE, Kiehn TE, Armstrong D. Bacterial resistance in the patient with neoplastic disease. Infect Dis Clin Pract 1995; 4:13644
Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Morb Mortal Wkly Rep 1995; 44(RR-12):113
deLalla F. Antibiotic treatment of febrile episodes in neutropenic cancer patients: clinical and economic considerations. Drugs 1997; 53:789804
Elting LS, Bodey GP, Keefe BH. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis 1992; 14:12017
European Organization for Research and Treatment of Cancer (EORTC) International Antimicrobial Therapy Cooperative Group, National Cancer Institute of CanadaClinical Trials Group. Vancomycin added to empirical combination antibiotic therapy for fever in granulocytopenic cancer patients. J Infect Dis 1991; 163:9518
Feld R, DePauw B, Berman S, et al. Meropenem versus ceftazidime in the treatment of cancer patients with febrile neutropenia: a randomized, double-blind trial. J Clin Oncol 2000; 18:36908
Hughes WT, Armstrong D,Bodey GP,Bow EJ,Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KVI, Shenep JL, Young LS; 2002 Guidelines for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer Clinical Infectious Diseases 2002;34:730-751
International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Ann Intern Med 1993; 119:58493
Klastersky J, Paesmans M, Rubenstein EB, et al. The multinational association for supportive care in cancer risk index: a multinational scoring system for identify low-risk febrile neutropenic cancer patients. J Clin Oncol 2000; 18:303851
Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based clinical practice guidelines. J Clin Oncol 2000; 18:355885
Pizzo PA, Ladisch S, Robichaud K. Treatment of gram-positive septicemia in cancer patients. Cancer 1980; 45:2067.
Ramphal R. Is monotherapy for febrile neutropenia still a viable alternative? Clin Infect Dis 1999; 29:50814
Rolston KVI, Tarrand JJ. Pseudomonas aeruginosastill a frequent pathogen in patients with cancer: 11-year experience at a comprehensive cancer center. Clin Infect Dis 1999; 29:4634
Winston DJ, Ho WG, Bruckner DA, Champlin RE. Beta-lactam antibiotic therapy in febrile granulocytopenic patients: a randomized trial comparing cefoperazone plus piperacillin, ceftazidime plus piperacillin, and imipenem alone. Ann Intern Med 1991; 115:84959.