Introduction
Lead is a highly toxic material with cumulative effect.1,2 There is a daily
intake of these materials via food, water, as well as evaporating chemical
compounds in air which can enter human organism by breathing. According to its
physically-chemical features, lead is similar to calcium, so that Pb2+ ions
can, in isomorphic way, replace Ca2+ ions in the structure of hydroxyapatite
(3), so the enlarged content of this material may be expected in mineral
tissues-bones and teeth.1,2 During the physiological processes of remodeling of
bony tissue, a part of Pb2+ ion may migrate, via oral and biological fluids, to
other organs-brain, kidneys, liver. This may be particularly im-portant for
women during pregnancy and lactation, when mineral tissues (teeth and bones)
become endogenic source of lead.
According to the literary data, lead content in teeth starts from 1,5 µg/g to
65µg/g,4-8 which depends on the tooth type, patient's age, and
socio-geographical dwelling conditions. As far as literature is concerned, we
found no data about the possibilities of secretions of lead under the influence
of oral fluids, food the teeth are daily exposed to.
Metal-ceramic crowns that are implanted into a living tissue, out of either
health or aesthetic reasons, are constantly exposed to corrosive influence of
food and drink. There-fore, these implants should fulfill certain conditions:
that they don't have toxic, allergic or mutagenic effect on tissues; that they
are electro-chemically stable and resistant to corrosive outdoor effects.
However, these materials, due to the improvement of their features, color,
control of some physically-chemical processes present during construction of
implants, are added some heavy metals and their compounds.9 There may occur some
unremoved dirt in the course of implants construction and preparation, which in
turn can be another source of heavy metals in final prosthesis products. In
order to claim that dental implants have no toxic effect on organism, an
effective control is required, as it is the only way to increase the protection
and lessen the risk of disease development in humans come into being.
The overall content of lead, evaporated in 24 hour time in 4% CH3COOH from
natural teeth-intact and implants, was determined by potentiometric stripping
analysis (PSA) along with oxygen as an oxidant. This analytic technique was
chosen as highly sensitive, selective, quick, reproductive, with quite cheaper
instrumentation whose results match with the results of inflammable AAS
technique.10-12
The aim of the study
The aim of this paper was to determine the overall content of lead in matrix
teeth and metal-ceramic crowns. Moreover, it aimed at determining the
percentage of lead, from teeth and corresponding implants that can be
transported through organism to organs lead had affinity to kidneys, brain,
liver. This was based on the lead content that migrated in 4% CH3COOH during the
period of 24 hours at normal indoor temperature.
Material and method
The samples in this paper were metal-ce-ramic crowns and intact teeth with
amalgam filling obtained at Clinic of Stomatology in Nis. The amalgam filling
was removed from the tooth using mechanical method; so, these teeth were
analyzed in the same way as all the other samples. Metal-ceramic crowns were
prepared according to a usual routine that is applied in prosthesis praxis,
which included a range of physically-chemical processes. Teeth and metal-ceramic
crowns were, after cleaning and chopping dissolved with 5 cm3 69% HNO3 and 7cm3
37% HCI. The mixtures were gradually being heated up to 130OC, which was being
maintained during the mineralization lasting 2 hours. The solutions containing
sediment were filtrated through filter paper Whatman No 541, whereas filters
were being dissolved up to 100 cm3 4% CH3COOH (pH 2,5). At the same time mass of
these samples was determined having been treated with freshly prepared 4%
CH3COOH (100 cm3) during a 24 hour period aiming at determining lead soluble
content. Both total and soluble lead content were determined by PSA and
inflammable AAS. PSA is a specific analytic technique mostly used for
determining metal traces, that is, metal ions. It almost fully complies with
very rigid general and specific micro- analytic demands, and its most prominent
features are the following: high sensitivity (up to 10 -11 mol/dm3); a very good
analytic selectivity; the possibility of determi-ning greater number of elements
at the same time; the possibility of unlimited repetition of the same solution
analysis, lower price of instrumentation, and its exploitation in relation to
competing techniques.12-15
PSA modification with oxygen as an oxidance along with diffusion conditions of
mass transmission during the analytic pace were applied in this work. This PSA
modification is the easiest as it uses already existing dissolved oxygen as
oxidant means, which lessens the risk of contamination applying other oxidant
means.
The volume of 25 cm3 sample was used for PSA, and it was analyzed without being
dissolved. Optimal, experimental conditions for PSA of lead defined earlier,
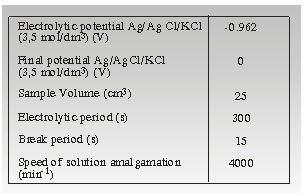
were shown in Table 1.
Table1. Experimental conditions for determining Pb (II) using PSA
Lead content in solutions was determined by inflammable AAS technique, following
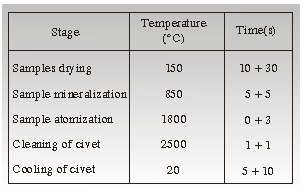
the stages shown in Table 2.
Table 2. Stages for determining of lead by flammable AAS technique
The sample volume for all the analyses was from 20 mm3. The analyses were carried
out in an internal atmosphere of argon, while the flow of argon through civet
was 300 cm3/min.
Results and discussion
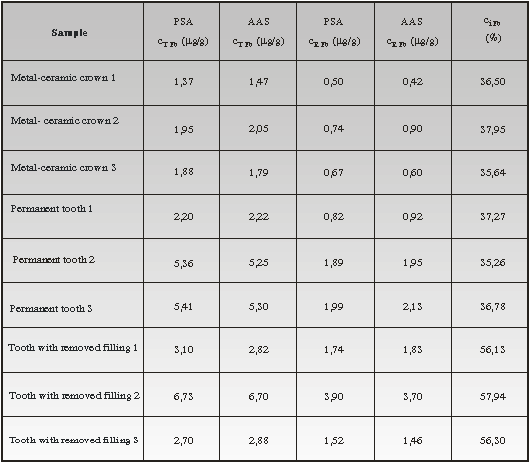
The results of determining total and soluble lead content from intact teeth and
corresponding implants obtained by applying PSA and inflammable AAS technique
were given in Table 3. All values were shown as a mead value of five
measurements.
Total lead content in intact teeth were ranging from 2,20 to 5,41µg/g and it is
in accordance with the interval values that can be found in literary data.4,5
However, the researches carried out by Gulson and his associates6,7,8 pointed
out that the total lead content in teeth had been rather higher (reaching up to
63 µg/g). The evaporation from intact teeth applying 4% CH3COOH (pHa 2,5) is
ranging from 0,82 to 1,99 µg/g, which in relation to the overall content of this
material amounts around 40%.
The overall lead content in metal-ceramic crowns was ranging from 1,37 to 1,95
µg/g, whereas the soluble lead content was from 0,5 to around 0,8 µg/g. However,
disregarding the total lead content, the percentage of lead released from both
metal-ceramic crowns and intact teeth, in 4% CH3COOH, approximately equals 40%.
These results indicate that regardless of the fact that the percentage of
soluble lead from certain dental ceramic components ceramic powder, colors,
steal12 is different (from 1% to 70%), in the end, in the final product, implant
percentage corresponds to intact tooth. The usual way of implant construction,
used in stomatological-prosthesis praxis, and which in turn includes numerous
physically-chemical processes (hydration, crystallization, recry-stallization,
ionic change…) enables the crown construction which, being treated with 4%
CH3COOH and mixture HNO3 and HCI, behaves as intact tooth in accordance with
the availability of heavy metals as well as the possibility of extraction.
The percentage of soluble lead from the tooth the amalgam filling had been
implanted in, was higher, around 60%. The higher content of released lead from
these teeth is a consequence of the total, overall content, as well as the tooth
treatment. In these teeth, on the spot where filling used to be, enamel was
scraped so that Pb2 ions leave the primary matrix more easily.
The soluble lead content from metal-ceramic crowns ranging from 0,50 up to
0,74 µg/g and teeth ranging from 0,82 to 1,99 µg/g, especially the teeth out of
which the filling was removed 1,46 to 3,70 µg/g, should not be neglected, having
in mind that it is rather toxic metal which is transported by oral and
biological fluids throughout the whole organism and which accumulates in vital
organs - brain, kidneys and liver.2
Table 3. Total and soluble lead content from intact teeth and metal-ceramic
crowns,
determined by PSA and AAS
Conclusion
According to the analyzed results, the following may be emphasized:
- in less acid medium, 4% CH3COOH pH a 2,5, in a 24 hour period at the indoor
tem-perature, around 40% of lead is released from intact teeth, around 2,5 to
5,5µg/g of the total highly toxic metal content.
- under the same conditions, out of teeth that used to have implanted amalgam
filling, around 60% of lead is secreted from the total content (from 3 to 7
µg/g)
- out of metal-ceramic crowns, disregarding their good aesthetic, mechanical and
biological chemical features, around 40% of lead is secreted, which is not so
harmless since around 0,5 to 1µg/g of highly toxic metal, lead with cumulative
effect is released during a 24 hour period in acid medium (4% CH3COOH, pHaa
2,5).
In long time period, those quantities of released lead, both from teeth and
dental implants respectively, should not be neglected having in mind the
detrimental and toxic effect of this metal in organism.
The results of this study show that PSA can successfully be applied for
determining low lead contents in intact teeth implants as well as
stomatological- prosthesis implants, which is indicated as well by the precise
matching of the results of PSA with inflammable AAS technique. AAS technique is
advisable to be used in standards for determining low metal content (ISO
7086/2); however, the price of its instrumentation and exploitation is
considerably higher in relation to PSA, including all the other already quoted
advantages of this technique, as well as the fact that it is the instrument of
domestic construction and production (STRIPING ANALYZATOR M1).
Conclusion
-
Goyer RA, Bingham E, Cohrssen B, Powell CH. Patty's Toxicology, 2001. -
Toxicological profile for lead, U.S. Department of health and human services. Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, 1999.
-
Crisp S, Jennings AM, Wilson DA. A study of temperature changes occuring in setting dental cements. J Oral Rehab 1987; 5: 139-144.
-
Bayo J, Moreno-Grau S, Martinez M J, Moreno J, Angosto JM, Moreno-Clavel J, Guillen Perez JJ, Garcia-Marcos L. Environmental and physiological factors affecting lead and cadmium levels in deciduous teeth. Arch Environ Contam Toxicol 2001; 41: 247-254.
-
Oehme M, Lund W, Jonsen J. The determination of copper, cadmium and zinc in human teeth by anodic stripping voltammetry. Anal Chim Acta 1978; 100: 389-398.
-
Gulson BL. Contribution of tissue lead to blood lead in adult female subjects based on stable lead isotope methods. J Lab Clin Med 1995;125: 703-712.
-
Gulson BL, Wilson D. History of lead exposure in children revealed from isotopic analyses of teeth. Arch Environment Health1994; 49: 279-283.
8. Gulson BL, Gillings BR. Lead exchange in teeth and bone-a pilot study using stable lead isotopes. Environ Health Perspect 1997; 105: 820-824. -
Vujošević Lj, Stamenković D, Obradović-Đuričić K, Pavlović G, Popović G. Stomatološki materijali, Medicinska knjiga, Beograd, 1997.
10. Kaličanin B, Marjanović N, Suturović Z. Determination of the soluble lead in the glassware by the poten-tiometric stripping analysis. APTEFF 2001; 32: 61-70. -
Kaličanin B, Marjanović N, Suturović Z. Appli-cation of potentiometric stripping analysis with constant inverse curent in the analitic step for determining lead in glassware. J Serb Chem Soc 2002; 67: 213-220.
-
Nikolić R, Kaličanin BM, Marjanović N. Poten-tiometric stripping analysis of the soluble lead released from dental ceramic materials. Facta Universitatis, series: Physics, Chemistry and Technology 2001; 2: 159-163.
-
Suturović Z. Elektrohemijska striping analiza, Tehnološki fakultet, Novi Sad, 2003.
-
Jagner D, Sahlin E, Ratana-Ohpas R, Axelsson B. Rapid method for the determination of copper (II) and lead (II) in tap water using a portable potentiometric stripping analyser. Anal Chim Acta 1993; 278: 237-242.
-
Suturović ZJ, Marjanović NJ, Dostanić NM. Potentiometric stripping analysis of lead in vinegars: development of a method. Nahrung 1997; 41: 111-113.
-
Nikolić R, Kaličanin BM. 3th International Con-ference of the Chemical Societies of the South-Eastern European Countries on Chemistry in the New Mille-nnium-an Endless Frontier, Bucharest, PO 185, 2002.
Adress for correspondence:
Biljana Kaličanin
Faculty of Technology
Leskovac 16000
Serbia and Montenegro
- Copyright © 2003 by The Editorial Council of The Acta Stomatologica Naissi